Abstract
Methotrexate (MTX) is an anti-metabolite in cancer chemotherapy and is associated with various toxicities assigned to inflammation and oxidative stress. The present study was undertaken to corroborate the therapeutic effects of bone marrow mesenchymal stem cells (BM-MSCs) and adipose-derived mesenchymal stem cells (AD-MSCs) in MTX-induced intestinal toxicity in experimental animals as compared with dexamethasone (Dex). Rats were divided into five groups: I-Normal control group, II- MTX (14 mg/kg, as a single dose/week for 2 weeks), III & IV- BM-MSCs & AD-MSCs (2 × 106 cells/rat, 1 week after last dose of MTX), respectively, plus V- Dex (0.5 mg/kg/ for 7 days, 1 week after last dose of MTX). MTX induced marked intestinal elevation of interleukin-6, total oxidant, and nitrite/ nitrate, caspase-3 contents and myeloperoxidase activity, along with the reduction of reduced glutathione content and catalase activity. In conclusion, the positive modulation of MTX toxicity could be attributed to the free radical scavenging, anti-inflammatory and antiapoptotic potential of BM-MSC and AD-MSCs which will possibly make them as remarkable hopeful for the treatment of intestinal injury.
Author Contributions
Academic Editor: Jun Wan, Department of Medical and Molecular Genetics, Indiana University School of Medicine, USA.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Marwa A. Masoud, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Methotrexate (MTX) is an antagonist of folic acid 1. MTX competitively inhibits the dihydrofolate reductase (DHFR) enzyme, which participates in the synthesis of folic acid and inhibits conversion of dihydrofolate to tetrahydrofolate. Subsequently, DHFR is essential for biosynthesis of thymidine and purines, which are needed for synthesis of DNA. Blockade of tetrahydrofolate synthesis by MTX leads to inability of cells to divide and to produce proteins 2. Subsequently, MTX is used as an antimetabolite in cancer chemotherapy in the treatment of lymphocytic leukemia, non-hodgkin’s lymphoma, osteosarcoma, head neck cancer, and mammary gland tumors 3. Moreover, MTX is also intervened for rheumatoid arthritis and refractory inflammatory disease treatments 4.
Nevertheless, MTX is restrained by its toxicity, including intestinal injury causes severe mucositis 5, enterocolitis, cardiotoxicity, nephrotoxicity and hepatotoxicity. Mucositis involves inflammation and mucosal ulceration of the alimentary tract, resulting in symptoms including pain, abdominal bloating, nausea, vomiting diarrhea and weight loss and disrupted chemotherapy 6, 7. In addition, chemotherapy may exert cell damaging or a cell-destroying effect through the generation of reactive oxygen species, or through enzymatic or transcription factors (NF-ƘB) which leads to up regulation of genes responsible for production of proinflammatory cytokines TNF-a, IL-1β and IL-6. This leads to tissue injury and apoptosis 8.
Bone-marrow mesenchymal stem cells (BM-MSCs) are fibroblast-like, pluripotent adult stem cells. BM-MSCs can adhere to plastic and grow readily in the laboratory producing other types of cells, including new stem cells identical to mother cells. MSCs have been shown to have immunomodulatory capabilities due to the secretion of several growth factors 9. BM-MSCs reduce intestinal injury in rats 10. Previous study demonstrated that MSCs can beneficially produce paracrine growth factors and anti-inflammatory cytokines 11. It should be noted that MSCs respond to TNF-α, but do not produce TNF-α 12.
Adipose-derived mesenchymal stem cells (AD-MSCs) seem to be a promising regenerative therapeutic agent due to the minimally invasive approach of their harvest and multi-lineage differentiation potential. The harvested adipose tissues are further digested to extract stromal vascular fraction, which is cultured, and the anchorage-dependent cells are isolated in order to characterize their stemness, surface markers, and multi-differentiation potential 13, 14, 15, 16.
Dexamethasone (Dex) is a well-known steroid agent that regulates inflammation by downregulates expression of anti-inflammatory mediators such as TNF-α, IL-6 by decreasing the mRNA stability of these cytokine 17.
The present study aimed to study the therapeutic effects of BM-MSCs and AD-MSCs in MTX-induced intestinal injury in rats as compared with Dex.
Materials and Methods
Animals
Male Wistar albino rats, weighing 150-200 g, obtained from the animal house of the National Organization for Drug Control and Research (NODCAR, Cairo, Egypt) were used in the present study. Animals were housed for at least one week in the laboratory room prior to testing under controlled environmental conditions; constant temperature (25 ± 2 ͦ C), humidity (60 ± 10%), and alternating 12 h light/dark cycles. Standard pellet diet and water was allowed ad libitum. The investigation was complied with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) 18.
Experimental Design
Forty rats were randomly allocated into 5 groups. Group 1 received normal saline orally and served as control group. The rest of animals received MTX (Orion Pharma, Finland) orally in a dose 14 mg/kg/week for 2 consecutive weeks 19. Rats of the second group were left untreated, while groups 3 and 4 were intraperitoneally injected with 2×106 cells in 500 μl PBS/ rat of BM-MSCs 20 and AD-MSCs 21, respectively, 1 week after the last dose of MTX. The last group was treated with Dex (0.5 mg/kg, p.o; Amriya Pharm, Egypt) for 7 days starting 1 week after the last dose of MTX 22. Rats were euthanized by decapitation after six weeks from the beginning of the experiment; intestine was immediately excised, washed with ice-cold saline, and blotted dry. Intestinal tissues were homogenized in PBS (10% w/v), centrifuged (4,000 rpm, 4°C, 15 min), supernatants were frozen at −80°C for further assessment.
Isolation of BM-MSCs from Rats
Bone marrow was harvested by flushing the tibiae and femurs of 6 weeks old male white albino rats, with Dulbecco’s modified Eagle’s medium (DMEM,GIBCO/BRL) supplemented with 10% fetal bovine medium (GIBCO/BRL). Nucleated cells were isolated with a density gradient Ficoll/Paque (Pharmacia) and resuspended in complete culture medium supplemented with 1% penicillin-streptomycin (GIBCO/BRL).
Cells were incubated at 37°C in 5% humidified CO2 for 12-14 days as primary culture or upon formation of large colonies. When large colonies developed (80-90% confluence), cultures were washed twice with phosphate buffer saline (PBS) and cells were trypsinized with 0.25% trypsin in 1mM EDTA (GIBCO/BRL) for 5 minutes at 37°C. After centrifugation (at 2400 rpm for 20 minutes), cells were resuspended with serum-supplemented medium and incubated in 50 cm2 culture flask (Falcon). The resulting cultures were referred to as first-passage cultures 23. Mesenchymal stem cells in culture were characterized by their adhesiveness and fusiform shape and by flow cytometric detection of cluster of differentiation (CD) 29, one of surface marker of rat mesenchymal stem cell 24.
Isolation of AD-MSCs
Adipose tissue was excised from the inguinal fat pad (i.e., subcutaneous) under complete aseptic condition. Then adipose tissue underwent enzymatic digestion by 0.075% collagenase II (sigma) in Hank's Balanced Salt Solution for 60 minutes at 37°C with shaking. Digested tissue was filtered and centrifuged, and erythrocytes were removed by treatment with erythrocyte lysis buffer. The remaining cells were transferred to tissue culture flasks with Dulbecco Modified Eagle Medium (DMEM; Gibco/Invitrogen Corp., Grand Island, NY) plus supplement F12 (Gibco/Invitrogen). After an attachment period of 24 hours, non-adherent cells were removed by a phosphate buffered solution (PBS; Gibco/Invitrogen) wash. Attached cells were cultured in DMEM/F12 media, supplemented with 10% fetal bovine serum (FBS; Gibco/Invitrogen), 0.1 µM dexamethasone (Sigma-Aldrich), streptomycin (Gibco/Invitrogen), and 1.25 mg/L amphotericin B (Gibco/Invitrogen), and expanded in vitro until passage three 25.
Morphological Identification of MSCs
Mesenchymal stem cells in culture were characterized by their adhesiveness and fusiform shape and by flow cytometric detection of cluster of differentiation (CD) 29, one of surface marker of rat mesenchymal stem cell 24.
Determination of Intestinal Inflammatory Markers; myeloperoxidase (MPO) Activity and Interleukine-6 (IL-6) Contents
Determination of intestinal MPO activity was done using a kinetic colorimetric method described by Bradley et al.26. In brief, firstly prepared substrate by preparing 100ml of 50mM K2Hpo4 then pH was adjusted to 6 then mixed with 16.7 mg of ortho-dianisidine. Finally, 0.17ml of 30% of H2O2was added to the mixture. The intestinal homogenate was centrifuged after 3 cycles of freezing and thawing (-700C/370C) to 12000 rpm for 15 minutes at 4OC then 50µL of supernatant was mixed with 2.4 ml of substrate. Absorbance was read at 460 nm after 30 seconds for every 30 seconds for 2 minutes.
MPO was calculated in terms of (U/g) =
where; ΔA= change of the sample absorbance over 2 minutes.
Besides, interleukien-6 (IL-6) was determined quantitatively by ELISA using a test reagent kit (SinoGeneClon Biotech Co., Ltd; China) according to the manufacturer’s instructions. Intestinal IL-6 contents were estimated in the tested samples and determined as pg/g tissue from the standard curve constructed.
Determination of Intestinal Oxidative Stress Markers; Total Oxidant (TO), Reduced Glutathione (GSH), and Nitrite/Nitrate (NOx) Contents along with Catalase (CAT) Activity
The total oxidant (TO) content of samples was determined as previously described in 27. In addition, determination of intestinal reduced glutathione (GSH) was done according to the described method by Beutler et al. 28 and expressed as mg/g wet tissue. Additionally, vanadium trichloride was used to reduce nitrate to nitrite according to Miranda et al.29 in nitric oxide assay. The method of nitrite estimation is based on Griess reaction that was performed using the kit provided by Biodiagnostic (Giza, Egypt). Furthermore, catalase activity was assessed in tissue homogenate by the kinetic method of Cohen et al.30 using the kit provided by Biodiagnostic (Giza, Egypt).
Determination of Intestinal Caspase-3 Content
Activity of caspase3 was determined using the caspase-3 colorimetric assay Kit (R&D systems, a bio-techne brand, Minneapolis, USA) according to the instructions of the manufacturer. Actually, cells that are suspected to or have been induced to undergo apoptosis are first lysed to collect their intracellular contents. The cell lysate can then be tested for protease activity by the addition of a caspase-specific peptide that is conjugated to the color reporter molecule p nitroaniline (pNA). The cleavage of the peptide by the caspase releases the chromophore pNA, which can be quantitated spectrophotometrically at a wavelength of 405 nm. The level of caspase enzymatic activity in the cell lysate is directly proportional to the color reaction 31.
Statistical Analysis
The obtained results were presented as mean ± standard error of the mean. Comparisons between means were carried out using One-Way ANOVA followed by Tukey-Kramer multiple comparisons test. Statistical analysis was performed using GraphPad Prism software (version 5); a probability level of less than 0.05 was accepted as statistically significant.
Results
Identification of MSCs
Isolated and cultured undifferentiated MSCs were typical of adherent spindle and fibroblast-like morphology and reached 70-80% confluence at 2 weeks culture (Figure A), in addition flowcytometric analysis of the MSCs showed that these cells were positive for surface marker CD29 (Figure B).
Figure (A&B).Typical morphological aspects of MSCs where they were identified by their fusiform fibroblast-like structure (A); and flowcytometric characterization analysis; showing cells that were uniformly positive for CD29 (B).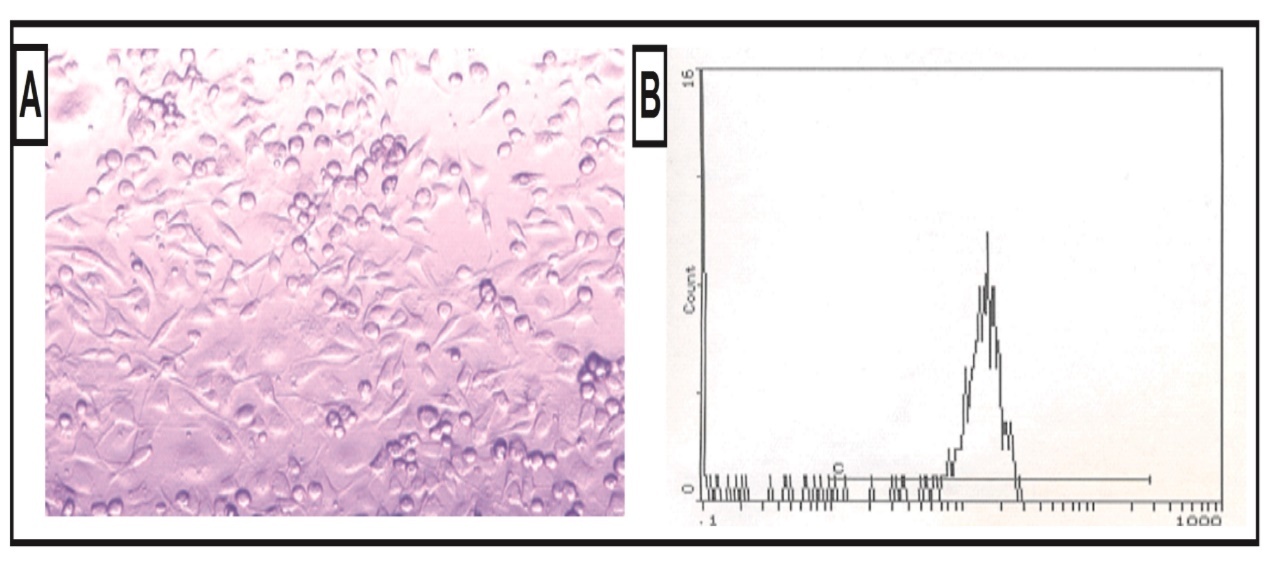
The effects of BM-MSCs and AD-MSCs on inflammatory markers as compared with Dex in MTX-induced intestinal injury in rats
Administration of MTX produced a significant increase in intestinal content of MPO (0.755 ± 0.047, to 3 folds) as well as IL-6 (115 ± 3.59, to 4 folds) as compared with the control group (0.186 ± 0.0177) and (23.7 ± 1.99), respectively (Figure 1). In contrast, either treatment with BM-MSCs or AD-MSCs or Dex decreased intestinal contents of MPO (0.107 ± 0.010, by 86%), (0.221 ± 0.03, by 71%), and (0.0942 ± 0.004, by 87%), respectively, as well as IL-6 (25.6 ± 1.75, by 78%), (16.5 ± 1.37, by 86%), and (14.4 ± 1.82, by 87%), respectively, as compared to MTX group.
Figure 1.Effect of treatment with BM-MSCs or AD-MSCs on IL-6 content (A) and myeloperoxidase activity (B) as compared with Dex in MTX-induced intestinal injury in rats.
The effects of BM-MSCs and AD-MSCs on oxidative stress markers as compared with Dex in MTX-induced intestinal injury in rats
Administration of MTX produced a significant increase in intestinal content of proxidant, TO, (148 ± 7.17, to 3 folds) plus NOx (358 ± 18.7, to 1 fold) accompanied with significant reduction in the enzymatic antioxidant activity of CAT (0.199 ± 0.008, by 70%) and non-enzymatic antioxidant content of GSH in the intestine (0.150 ± 0.027, by 83%) compared with the control group (39.6 ± 3.25), (173 ± 10.4), (0.641 ± 0.046), and (0.895 ± 0.137), respectively.
In contrast, either treatment with BM-MSCs, AD-MSCs or Dex decreased intestinal contents of TO (70.5 ± 6.95, by 52%), (74.1 ± 5.39, by 50%), and (63.2 ± 2.61, by 57%), respectively, NOx (125 ± 13.1, by 65%), (166 ± 10.4, by 54%), and (110 ± 10.6, by 70%), respectively, accompanied with significant increase intestinal CAT activity (0.510 ± 0.0263, by 150%) , (0.339 ± 0.009, by 70%), (0.278 ± 0.01, by 40%), respectively, as well as GSH content (0.994 ± 0.127, to 6 folds), (0.651 ± 0.01, to 3 folds), and (0.855 ± 0.026, to 5 folds), respectively, compared to MTX group (Figure 2 & Figure 3).
Figure 2.Effect of treatment with BM-MSCs or AD-MSCs on proxidant content (A) and nitrite/nitrate content (B) as compared with Dex in MTX-induced intestinal injury in rats.
Figure 3.Effect of treatment with BM-MSCs or AD-MSCs on catalase activity (A) and reduced glutathione content (B) as compared with Dex in MTX-induced intestinal injury in rats.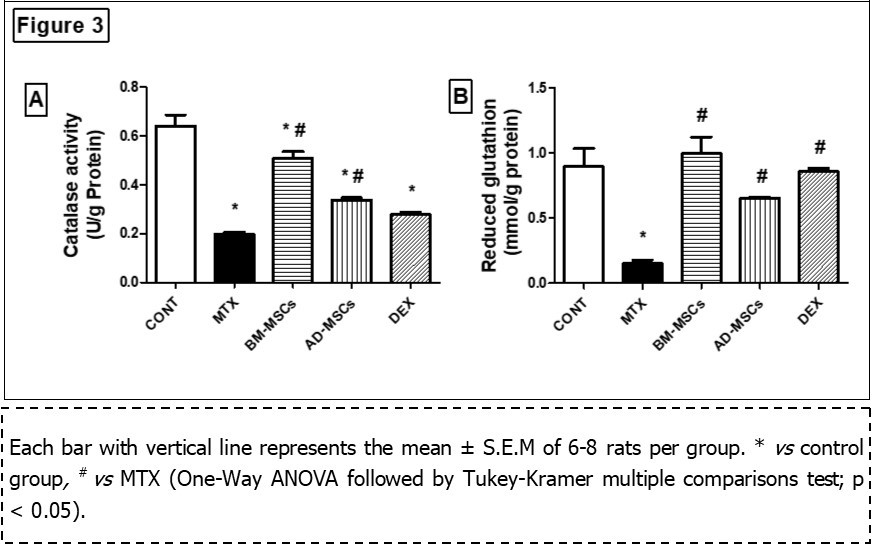
The effects of BM-MSCs and AD-MSCs on (apoptotic marker) caspase-3 as compared with Dex in MTX-induced intestinal injury in rats
Administration of MTX produced a significant increase in apoptotic content of Caspase-3 (2.33 ± 0.211, to 1 fold) as compared with the control group (Figure 4). In contrast, either treatment with BM-MSCs or AD-MSCs or Dex decreased intestinal contents of Caspase-3(1.00 ± 0, by 57%), (1.33 ± 0.211, by 43%), and (1.33 ± 0.211, by 43%), respectively, as compared to MTX group.
Figure 4.Effect of treatment with BM-MSCs or AD-MSCs on caspase-3 as compared with Dex in MTX-induced intestinal injury in rats.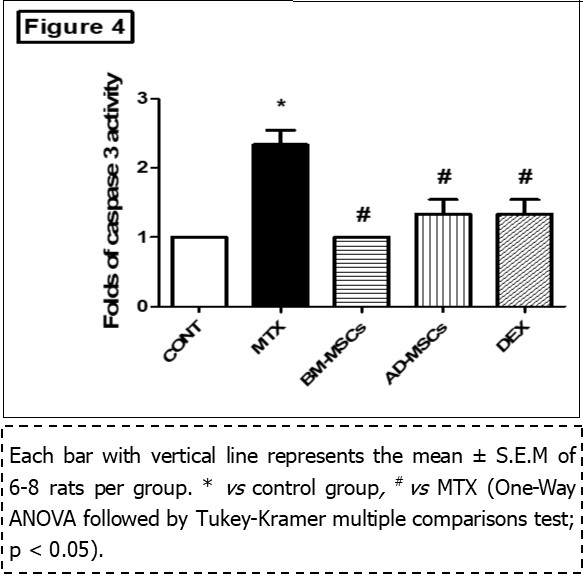
Discussion
This study investigated the anti-apoptotic and antioxidant effects of two different types of mesenchymal stem cells (MSCs), BM-MSCs and AD-MSCs against MTX-induced intestinal injury (mucositis) as compared to Dex. The main mechanism behind the development of mucositis was considered to be the result of direct cytotoxic effects of chemotherapy or radiotherapy on the basal cells of the epithelium because of its high cell turnover rate 32, 33. Intestinal mucositis is one of the major problems in the patients receiving cancer treatment as MTX 34.
The present study confirmed that as MTX induced elevation in intestinal TO, and NOx contents, MPO, IL-6 and apoptotic marker caspase-3, in addition; it caused depletion of antioxidant defense GSH content and CAT activity as compared to control animals. This result in accordance with studies of Kolli et al. and Moghadam et al 6,35. who reported that oxidants such as malondialdehyde which is a product of lipid peroxidation and MPO which is the marker of neutrophil activation and infiltration increase, while the levels of non-enzymatic and enzymatic antioxidants such as GSH, CAT and superoxide dismutase (SOD) decrease in MTX induced small intestine mucositis by increasing the production of ROS 36, 37. Several studies have shown that sustained release of NO, as a result of iNOS up regulation, can lead to cellular damage and gut barrier failure 38. It may also be considered that MTX can inhibit some antioxidant enzymes which in turn may cause lipid peroxidation to increase due to a reduction in the activities of protective antioxidant enzymes such as SOD and catalase 39. In addition, recent advances showed that oxygen radicals and hydrogen peroxides (H2O2) are linked with the development of several pathological processes associated with chemotherapy, including adverse effects of antitumor drugs 40. MTX may most likely induce apoptosis through oxidative stress.
MTX is well established that pro-inflammatory cytokines, such as interleukin-1 (IL-1), tumor necrosis alpha (TNF-a) and interleukin-6 (IL-6) are potent inducers of iNOS in a wide variety of cells types, with consequent production of NO 41. Although the participation of pro-inflammatory cytokines in the intestinal mucositis has been shown 42, the role of nitric oxide is not fully understood. It has been demonstrated that nitric oxide is an important mediator of 5-florouracil (FU)-induced oral mucositis 43, suggesting that chemotherapy-induced nitric oxide synthase (iNOS) activation may play a critical role in mucosal injury.
This work revealed a marked antioxidant potential of BM-MSCs and AD-MSCs as shown by replenishing intestinal GSH content and CAT activity associated with hampering MPO and NO contents as compared to the MTX group. The antioxidant effects of stem cells were formerly demonstrated in various organs injury models 44, 45 through the down-regulation of nitric oxide metabolites 46. BM-MSCs administration improved GSH homeostasis probably via increased efflux of cysteine and GSH from tissues, and/or increased GSH synthesis and recycling 44. Notably, BM-MSCs inhibit the release of inflammatory mediators and lipid peroxidation 47. Recent studies have demonstrated that in vitro expanded MSCs of various origins have great capacity to modulate immune responses and change the progression of different inflammatory diseases 48.
Studies in intestinal injury in rodent models have demonstrated that MSCs can beneficially produce paracrine growth factors and anti-inflammatory cytokines to reduce intestinal injury in rats 10, 11. The results of this study suggest that MSCs can effectively reduce both the intestinal permeability and pathological damage associated with IR injury. MSCs have the potential for multidirectional differentiation. They participate in colonic mucosal regeneration 49.
In addition, the present study reported a prominent antioxidant as well as antiapoptotic effects for Dex. that probably exhibited elevated tissue GSH content and CAT activity in the settings of reduced MPO, TO, IL-6. Increasing of CAT activity may be by an enzymatic induction mechanism 50, as well as the attempt of intestinal tissue to counteract the increase of free radical generation 51. Dex administration declined mRNA expression of Caspase-3 as compared to MTX group. Dex was shown to suppress upregulation of pro-apoptotic factors, as well as and reduce caspase-3-like activity 52.
Conclusion
We have demonstrated that treatment with MTX induced intestinal epithelial damage in wild type rats. BM-MSCs and AD-MSCs reduced oxidative stress markers as compared with Dex in MTX-induced intestinal injury in rats. These findings suggest that the curative effects of BM-MSCs and AD-MSCs against MTX may rely on their anti-apoptotic function.
Acknowledgment
The authors are grateful to Dr. Laila Ahmed Rashed, Professor of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, for her efforts in isolation of mesenchymal stem cells.
References
- 1.RFC Leitao, GAC Brito, Oria R B, MBB Neto, EAL Bellaguarda. (2011) Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. , BMC Gastroenterol; 16, 11-90.
- 2.Howard S C, McCormick J, Pui C H, Buddington R K, Harvey R D. (2016) Preventing and Managing Toxicities of High-Dose Methotrexate. , Oncologist; 21(12), 1471-1482.
- 3.Hashkes P J, Becker M L, Cabral D A, Laxer R M, Paller A S. (2014) Methotrexate: new uses for an old drug. , J Pediat; 164(2), 231-6.
- 4.Cronstein B N. (2005) Low-dose Methotrexate: a mainstay in the treatment of rheumatoid arthritis. , Pharmacol Rev; 57(2), 163-72.
- 5.Chabner B, Wilson W, Supko J. (2007) Pharmacology And Toxicity Of Antineoplastic Drugs.Editorial:Lichtman,M.A.,Beutler,E.,Seligsohn,U.,Kipps,T.J.,Kaushansky,K. In Williams Hematology. 7th Edition 249-251.
- 6.Kolli V K, Abraham P, Isaac B, Kasthuri N. (2013) Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Dig Dis Sci;. 58(4), 959-969.
- 7.Sonis S T, Elting L S, Keefe D, Peterson D E, Schubert M. (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. , Cancer; 100(9), 1995-2025.
- 8.Logan R M, Stringer A M, Bowen J M, Yeoh A S, Gibson R J. (2007) The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. , Cancer Treat Rev 33, 448-460.
- 9.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B. (2006) Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease.Transplantation;. 81, 1390-1397.
- 10.Jiang H, Qu L, Li Y, Gu L, Shi Y. (2011) Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats.J Surg Res;. 168, 127-134.
- 11.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P. (2007) Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury.Am JPhysiolRenalPhysiol;. 292, 1626-1635.
- 12.LC van den Berk, Jansen B J, Siebers-Vermeulen K G, Roelofs H, Figdor C G. (2010) Mesenchymal stem cells respond to TNF but do not produce TNF.JLeukocBiol;. 87, 283-289.
- 13.Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galie M. (2008) Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem cells and development;. 17(5), 909-916.
- 14.H T Chen, Lee M J, Chen C H, Chuang S C, Chang L F. (2012) Proliferation and differentiation potential of human adipose‐derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. Journal of cellular and molecular medicine;. 16(3), 582-592.
- 15.Bräunig P, Glanzner W G, Rissi V B, PBD Gonçalves. (2018) The differentiation potential of adipose tissue-derived mesenchymal stem cells into cell lineage related to male germ cells. Arquivo Brasileiro de Medicina Veterinária e Zootecnia;. 70(1), 160-168.
- 16.Dubey N, Mishra V, Dubey R, Deng Y H, Tsai F C. (2018) Revisiting the advances in isolation, characterization and secretome of adipose-derived stromal/stem cells. International journal of molecular sciences;. 19(8), 2200.
- 17.Tobler A, Meier R, Seitz M, Dewald B, Baggiolini M. (1992) Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. , Blood; 79(1), 45-51.
- 18.Institute of LaboratoryAnimal Resources. Guide for the care and use of laboratory animals, 8th ed. , Washington, DC:NationalAcademyPress,1996
- 19.Coleshowers C L, Ukpong M OguntibejuOO, Truter E J. (2010) Effects Of Methotrexate On Antioxidant Enzyme Status In A Rodent Model. , Medical Technology SA; 24(1), 5-9.
- 20.Maron-Gutierrez T, Castiglione R C, Xisto D G, Oliveira M G, Cruz F F. (2011) Bone marrow-derived mononuclear cell therapy attenuates silica-induced lung fibrosis. The European respiratory journal;. 37(5), 1217-1225.
- 21.Fikry E M, Safar M M, Hasan W A, Fawzy H M, El-Denshary E E. (2015) Bone Marrow and Adipose‐Derived Mesenchymal Stem Cells Alleviate Methotrexate‐Induced Pulmonary Fibrosis in Rat: Comparison with Dexamethasone. Journal of biochemical and molecular toxicology;. 29(7), 321-329.
- 22.Chen F, Gong L, Zhang L, Wang H, Qi X. (2006) Short courses of low dose dexamethasone delay bleomycin-induced lung fibrosis in rats. European journal of pharmacology;. 536(3), 287-295.
- 23.Alhadlaq A, Mao J J. (2004) Mesenchymal stem cells: isolation and therapeutics. Stem cells and development. 13(4), 436-448.
- 24.Mohi El-Din MM, Rashed L A, Mahmoud Haridy MA, Khalil A M, Mohamed Albadry MA. (2017) Impact of bone marrow-derived mesenchymal stem cells on remodeling the lung injury induced by lipopolysaccharides in mice. , Future science OA 3(1), 162.
- 25.Tomiyama K, Murase N, Stolz D B, Toyokawa H.O'Donnell Det al.(2008) Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem cells. 26(2), 330-338.
- 26.Bradley P P, Priebat D A, Christensen R D, Rothstein G. (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. , Journal of Investigative Dermatology; 78(3), 206-209.
- 27.Erel O. (2005) A new automated colorimetric method for measuring total oxidant status. , Clinical biochemistry; 38, 1103-1111.
- 28.Beutler E, Duron O, Kelly B M. (1963) Improved method for the determination of blood glutathione. , J Lab Clin Med; 61, 882-888.
- 29.Miranda K M, Espey M G, Wink D A. (2001) A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite.Nitric Oxide;. 5(1), 62-71.
- 30.Cohen G, Dembiec D, Marcus J. (1970) Measurement of catalase activity in tissue extracts. , Analytical biochemistry; 34(1), 30-38.
- 31.El-Baz F K, Aly H F, Khalil W K, Booles H F. (2016) Neuroameliorative Effects Of Berry Extracts In Alzheimer Induced Rats. , Int J Pharm Bio Sci 7(4), 548-558.
- 32.Paris F, Fuks Z, Kang A. (2001) Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. , Science; 293, 293-297.
- 33.Sonis S T, Peterson R L, Edwards L J. (2000) Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. , Oral Oncol; 36, 373-381.
- 34.Hoekstra M, van Ede AE, Haagsma C J, MA van de Laar, Huizinga T W. (2003) Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. , Ann Rheum Dis; 62, 423-426.
- 35.Moghadam A R, Mohajeri D, Namvaran-Abbas-Abad A, Manafi H, Shahi D. (2013) Protective effect of turmeric extract on ethotrexate-induced intestinal damage and oxidative stress. , Chin J Nat Med; 11, 477-483.
- 36.Huang C, Hsu P, Hung Y, Liao Y, Liu C. (2005) Ornithine decarboxylase prevents methotrexate-induced apoptosis by reducing intracellular reactive oxygen species production. , Apoptosis; 10, 895-907.
- 37.Phillips D C, Woollard K J, Griffiths H R. (2003) The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. , Br J Pharmacol 138, 501-511.
- 38.Potoka D A, Nadler E D, Upperman J S, Ford H R. (2002) Role of nitric oxide and peroxynitrite in gut barrier failure. , World J Surg; 26(7), 806-811.
- 39.Cetinkaya A, Bulbuloglu E, Kurutas E B, Kantarceken B. (2006) N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. , Med Sci Monit; 12, 274-278.
- 40.Asvadi I, Hajipour B, Asvadi A, Asl N A, Roshangar L et al. (2011) Protective effect of pentoxyfilline in renal toxicity after methotrexate administration. Eur Rev Med Pharmacol Sci;. 15(9), 1003-9.
- 41.Estrada C, Gomez C, Martin C, Moncada S, Gonzalez C. (1992) Nitric oxide mediates tumor necrosis factor-alpha cytotoxicity in endothelial cells. , Biochem Biophys Res Commun; 186(1), 475-482.
- 42.Koning B A, Van Dieren JM, Lindenbergh-Kortleve D J, Sluis M Van der, Matsumoto T. (2006) Contributions of mucosal immune cells to methotrexate-induced mucositis. , Int Immunol; 18(6), 941-949.
- 43.Ribeiro R A, Bellaguarda E A, Macedo F D, Silva L R, Oria R B. (2007) Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hámster. , Cancer Chemother Pharmacol; 59(5), 603-12.
- 44.Iyer S S, Torres-Gonzalez E, Neujahr D C, Kwon M, Brigham K L. (2010) Effect of bone marrow-derived mesenchymal stem cells on endotoxin-induced oxidation of plasma cysteine and glutathione in mice. Stem Cells International;.
- 45.Penuelas O, Melo E, Sanchez C, Sanchez I. (2013) Antioxidant effect of human adult adipose-derived stromal stem cells in alveolar epithelial cells undergoing stretch. , Respir Physiol Neurobiol; 188(1), 1-8.
- 46.Lee S H, Jang A S, Kim Y E, Cha J Y. (2010) Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. , Respir Res; 11, 16.
- 47.Yang S, Piao J, Jin L, Zhou Y. (2013) Does pretreatment of bone marrow mesenchymal stem cells with 5-azacytidine or double intravenous infusion improve their therapeutic potential for dilated cardiomyopathy? Med Sci Monit Basic Res. 19, 20-31.
- 48.Ma S, Xie N, Li W, Yuan B, Shi Y. (2014) Immunobiology of mesenchymal stem cells. Cell death and differentiation;. 21(2), 216-225.
- 49.Valcz G, Krenács T, Sipos F, Leiszter K, Tóth K. (2011) The role of the bone marrow derived mesenchymal stem cells in colonic epithelial regeneration. , Pathol Oncol Res; 17, 11-16.
- 50.José H J, Berenice S G, Cecilia V R. (1997) Induction of antioxidant enzymes by dexamethasone in the adult rat lung. Life sciences;. 60(23), 2059-67.
Cited by (1)
- 1.I. Hegazy Marwa I. Hegazy, M. Asaad Aman, A. Rashed Lila, H. Ahmed Hanaa, 2022, Combined Treatment of Levetiracetam and Mesenchymal Stem Cells Reverses the Biochemical Aberrations in the Acute Phase of Epilepsy Induced by Pilocarpine in Rats, Biomedical and Pharmacology Journal, 15(1), 91, 10.13005/bpj/2346